CBSE Class 10 Science Notes Chapter 4 Carbon and its Compounds
Introduction:-
The amount of carbon present in the earth's crust and in the atmosphere is very less. The earth's crust has only 0.02% carbon in the form of minerals (like carbonates, hydrogen carbonates, coal and petroleum) and the atmosphere has 0.03% of carbon dioxide. Yet, we find that a large number of things that we use in our daily life are made of carbon compounds. Food, clothes, medicines, books and many other things are some examples.
The presence of carbon in a material can be tested by burning the substance in air and passing the gas formed through lime water. If the lime water turns milky, then the given material contains carbon.
Most carbon compounds are poor conductors of electricity and have low boiling and melting points, from which it can be concluded that the forces of attraction between these molecules are not very strong. Since these compounds are largely non-conductors of electricity, we can conclude that the bonding in these compounds does not give rise to any ions.
In the case of carbon, it has four electrons in its outermost shell and in order to attain noble gas configuration, it needs to either gain four electrons lose four electrons.
(1) It could gain four electrons forming C4- anion. But as the nucleus contains only six protons, it would be difficult for the nucleus to hold on to ten electrons.
(2) It could lose four electrons forming C4+ cation. But a large amount of energy would be required to remove four electrons leaving behind a carbon cation with six protons in its nucleus holding on to just two electrons.
Carbon overcomes this problem by sharing its valence electrons with other atoms of carbon or with atoms of other elements. Apart from carbon, there are many other elements also which form molecules by sharing electrons in this manner. The shared electrons 'belong' to the outer shells of both the atoms and lead to both atoms attaining the noble gas configuration.
Covalent Bond
The types of bonds which are formed by the sharing of an electron pair between two atoms are known as covalent bonds.
Depending upon the number of pairs of electrons shared between atoms, there can be single, double or triple covalent bonds.
Properties of Covalent Compounds
(1) Covalent compounds are usually Liquids or Gases.
Reason: This is due to the weak forces of attraction between their liquids or gases molecules.
(2) Covalent compounds have usually low melting and boiling points
Reason:- Covalently bonded molecules are seen to have strong bonds within the molecule, but intermolecular forces are small.
(3) Usually insoluble in water but the soluble in organic solvents
Reason:- This is also due to the presence of strong bonds within molecule and small intermolecular forces.
(4) Covalent compounds do not conduct electricity.
Reason:- Since the electrons are shared between atoms and no charged particles are formed, such covalent compounds are generally poor conductors of electricity.
Differences between lonic Bond and Covalent Bond
|
lonic Bond |
Covalent Bond |
|
1.An ionic bond is a chemical bond between two dissimilar (i.e. a metal and a non-metal) atoms in which one atom gives up an electron to another. 2. An ionic bond is formed between a metal and a non-metal. 3. Molecules have no definite shapes, as they have lattice structures. 4. Electrical and thermal conductivity is High. 5. Usually High melting point. 6. Usually highly soluble in water. 7. Usually solids at room temperature.
|
1. In a covalent bond the two atoms come together to share the electron, instead of an atom taking an electron from another.
2. A covalent bond is formed between two non metals that have similar electro-negativities.
3. Molecules have a definite shape.
4. No electrical conductivity but Thermal Conductivity is low. 5. Usually low Lower melting point. 6. lower solubility.
7. Exists as solids, liquids, gases |
Allotropes of Carbon:-
The various physical forms in which an element can exist are called allotropes of the element. Carbon exists in three solid forms called allotropes.
The three allotropes of carbon are:
(1) Diamond
(2) Graphite
(3) Fullerenes
Diamond:-
(1) Diamonds are colourless, transparent, sparkle and reflect light, which is why they are described as lustrous.
(2) It is extremely hard and has a high melting point.
(3) It does not conduct electricity.
Structure of diamond:
Diamond is one giant molecule of carbon atoms. Every atom in a diamond is bonded to its neighbours by four strong covalent bonds, leaving no free electrons and no ions. This explains why diamond does not conduct electricity.
Uses of diamond:
(1) Diamond is used in cutting instruments like glass cutters and in rock drilling equipment, as it is extremely hard.
(2) Diamonds are used for making jewellery.
(3) Sharp-edged diamonds are used by eye-surgeons as a tool to remove cataract.
Graphite:-
(1) Graphite is black, shiny and opaque.
(2) It is a very slippery material.
(3) Graphite is insoluble in water.
(4) It has a high melting point and is a good conductor of electricity, which makes it a suitable material for the electrodes needed in electrolysis.
Structure of graphite : Graphite contains layers of carbon atoms. In graphite, each carbon atom is bonded to three other carbon atoms in the same plane giving a hexagonal array. One of these bonds is a double-bond, and thus the valency of carbon is satisfied. Graphite structure is formed by the hexagonal arrays being placed in layers one above the other.
Uses of graphite:
(1) Powdered graphite is used as a lubricant for the fast moving parts of machinery.
(2) It is used for making electrodes in dry cells and electric arcs as it is a very good conductor of electricity
(3) It is used for making core of pencils called 'pencil leads.
Fullerenes:-
Fullerenes form another class of carbon allotropes. The first one to be identified was C-60 which has carbon atoms arranged in the shape of a football. Since this looked like the geodesic dome designed by the US architect Buckminster Fuller, the molecule was named fullerene.
VERSATILE NATURE OF CARBON
It is estimated that there are about three million carbon compounds whose formulae are known to chemists which is much greater than the compounds formed by all the other elements put together. The factors due to which this is possible in the case of carbon are:
Catenation:-
The property of carbon element due to which its atoms can join or link with one another to form long carbon chains is called catenation. These compounds may have long chains of carbon, branched chains of carbon or even carbon atoms arranged in rings.
In addition, carbon atoms may be linked by single, double or triple bonds
Tetravalency: -
The atomic number of carbon is 6 and its electronic configuration is 2.4. has a valency of 4, it can bond with four other atoms of carbon or atoms of other monovalent element. Carbon forms compounds with oxygen, hydrogen, nitrogen sulphur, chlorine and many other elements and these compounds have specific properties which depend on the elements other than carbon present in the molecule.
Strong Bonds due to Small Atomic Size:-
The bonds that carbon forms with most other elements are very strong due to its small size making these bonds very stable. This enables the nucleus to hold on to the shared pairs of electrons strongly. The bonds formed by elements having larger atoms are much weaker.
HYDROCARBONS
The compounds made up of hydrogen and carbon only are called hydrocarbons. These are the simplest organic compounds and all other compounds are considered to be derived from them by the replacement of one or more hydrogen atoms by other atoms or groups of atoms. The most important natural source of hydrocarbons is petroleum There are two types of hydrocarbons:
(1) Saturated hydrocarbons
(2) Unsaturated hydrocarbons
Saturated Hydrocarbons or Alkanes
(1) The hydrocarbons in which the carbon atoms are connected by only single bonds are called saturated hydrocarbons are alkanes.
(2) The general formula of saturated hydrocarbons are alkanes is C, H , where n is the number of carbon atoms in one molecule. The first few alkanes are methane (CH4), ethane (C2H6) and propane (C3H8 ).
(3) The saturated hydrocarbons are not very reactive.
(4) The saturated hydrocarbons generally give a clean flame. This is because the percentage of carbon is comparatively low which gets oxidized completely on combustion.
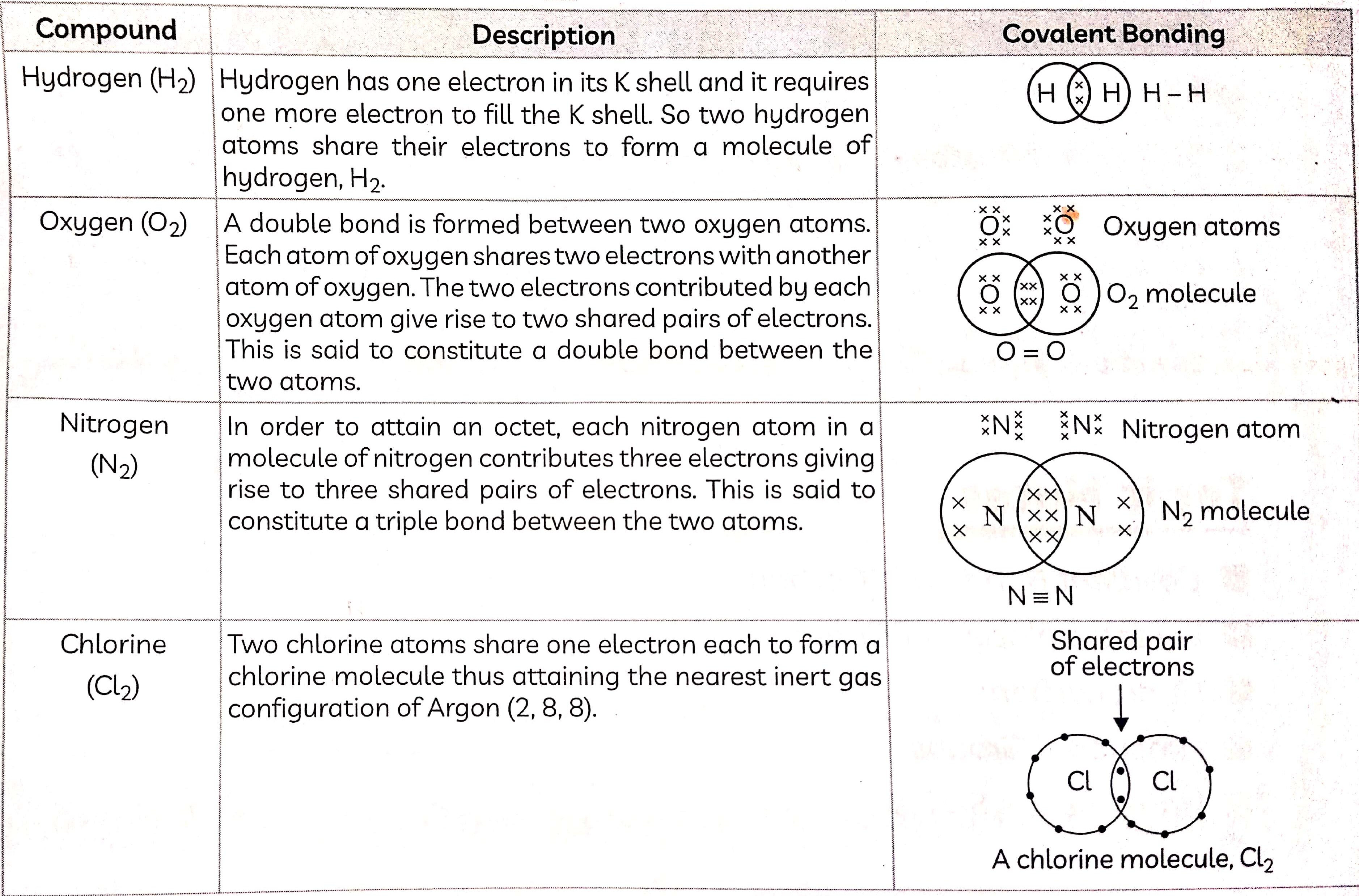
Structure of Saturated Hydrocarbons
The first step is to link the carbon atoms together with a single bond and then use the hydrogen atoms to satisfy the remaining valencies of carbon as shown in fig below
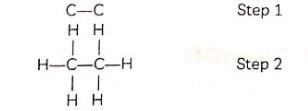
Methane: The simplest alkane is methane (CH4). Hydrogen has a valency of 1. As carbon has four valence electrons, carbon shares these electrons with four atoms of hydrogen in order to achieve noble gas configuration.
It is widely used as a fuel and is a major component of bio-gas and Compressed Natural Gas (CNG).
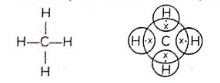
Ethane:
Ethane is an alkane having two carbon atoms. The molecular formula of ethane is C2H6. There are seven single covalent bonds present in one molecule of ethane - one covalent bond between the two carbon atoms and six single bonds between carbon and hydrogen atoms.
Unsaturated Hydrocarbons (Alkenes and Alkynes)
(1) The hydrocarbons in which the two carbon atoms are connected by a double bond or a triple bond are called unsaturated hydrocarbons.
(2) Unsaturated hydrocarbons may be alkenes (CnH2n ) or alkynes (CnH2n-2).
(3) The general formula of an alkene is (CnH2n ), where n is the number of carbon atoms in one molecule.
(5) The general formula of an alkyne is (CnH2n-2 ), where n is the number of carbon atoms in one molecule.
(6) These are more reactive than saturated hydrocarbons due to presence of double and triple
bonds which are the sites of chemical reactivity.
(7) These give a yellow flame with lots of black smoke. This is because the percentage of carbon is comparatively higher than saturated hydrocarbons which does not oxidize completely on combustion.
Structure of Unsaturated Hydrocarbons
In the first step two carbon atoms link together by single bond. Each carbon atom combines with two hydrogen atoms One valency per carbon atom remains unsatisfied which can be satisfied only if there is a double bond between the two carbon atoms.
Ethene:
The simplest alkene: Ethene is the simplest alkene having two carbon atoms and its molecular formula is C2H4. There is a double bond between the two carbon atoms and four single bonds between carbon and hydrogen atoms.
Ethyne-The simplest alkyne: The simplest alkyne is ethyne having two carbon atoms and its molecular formula is C2H2. There is a triple bond between the two carbon atoms and two single bonds between carbon and hydrogen atoms.
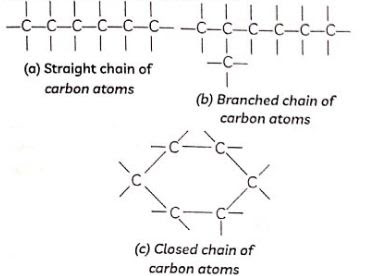
Chains, Branches and Rings
Cyclic Carbon atoms can form long 'chains' containing tens of carbon atoms. When carbon atoms combine three types of chains can be formed
(1) Straight chains
(2) Branched chains
(3) Closed chains or ring type chains
carbon atoms Some compounds have carbon atoms arranged in the form of a ring as in the case of cyclohexane (C6H12). Its structure is shown below. Similarly, the structure of benzene (C6H6) is also shown alongside
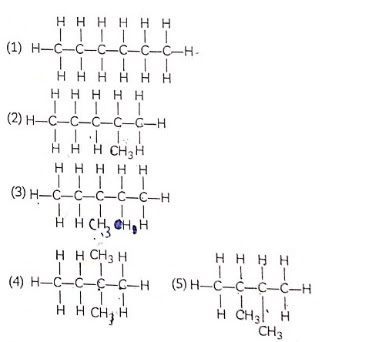
Structural Isomerism
Organic compounds having the same molecular formula but different physical and chemical properties due to different structures are called structural isomers and this property is called isomerism. Isomerism possible only with hydrocarbons having 4 or more carbon atoms.
If we look at the structure of butane (C4H10), we find that two different skeletons are possible with four carbon atoms having single covalent bond.
We find that both these compounds have the same molecular formula C4H10 but different structures and hence they called Give isomers.
Isomers of hexane:
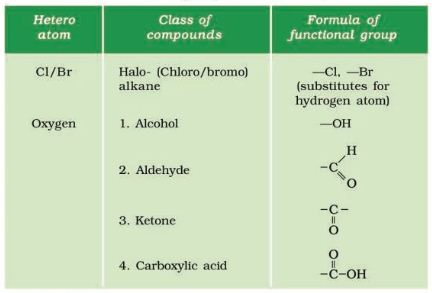
Functional group
A functional group in an organic compound is an atom or a group of atoms bonded together in a unique fashion, which is usually the site of chemical reactivity in an organic molecule. If in a hydrocarbon chain, one or more hydrogen atoms are replaced by atoms of other elements such as halogens, oxygen, nitrogen such that the valency of carbon remains satisfied, then the element replacing hydrogen is referred to as a heteroatom.
These heteroatoms confer specific properties to the compound, regardless of the length and nature of the carbon chain and hence are called functional groups.
Important Functional Groups
Some important functional groups are given in the Table below.
Free valency or valencies of the group are shown by the single line. The functional group is attached to the carbon chain through this valency by replacing one hydrogen atom or atoms.
Some More Examples of Functional Groups
HOMOLOGOUS SERIES
A homologous series is a group of organic compounds having similar structures and similar chemical properties in which the successive compounds differ by CH2 group.
Characteristics of Homologous Series
(1) All the members of a homologous series can be represented by the same general formula.
(2) Any two adjacent homologues differ by -CH2 or 1 carbon atom and 2 hydrogen atoms in their molecular formula.
(3) The difference in the molecular masses of any two adjacent homologues is 14 u.
(4) All the compounds belonging to the same homologous series have similar chemical properties since these are determined solely by the functional group.
(5) The members of a homologous series show a gradual change in their physical properties with increase in molecular mass. This is because the melting and boiling points increase with increasing molecular mass.
Related Topics:-
Chapter 1 Chemical Reactions and Equations - Notes
Chapter 2 Acids,Bases and Salts - Notes
Chapter 3 Metals and Non-metals -Notes
Chapter 4 Carbon and Its Compounds -Notes
Chapter 5 Periodic Classification of Elements -Notes
Chapterwise Notes for Class 10 Science Physics
Chapter 10 Light Reflection and Refraction -Notes
Chapter 11 Human Eye and Colourful World -Notes
Chapter 13 Magnetic Effects of Electric Current -Notes
Chapter 14 Sources of Energy -Notes
Chapterwise Notes for Class 10 Science Biology
Chapter 6 Life Processes -Notes
Chapter 7 Control and Coordination -Notes
Chapter 8 How do Organisms Reproduce? -Notes
Chapter 9 Heredity and Evolution -Notes
Chapter 15 Our Environment -Notes
Chapter 16 Management of Natural Resources -Notes
We hope the given -Notes for Class 10 Science will help you. If you
have any query drop a comment below and we will get back to you at
the earliest.












0 Comments
Please don't give write any spam link.